-
PDF
- Split View
-
Views
-
Cite
Cite
Luc De Meester, Wendy Van Doorslaer, Aurora Geerts, Luisa Orsini, Robby Stoks, Thermal Genetic Adaptation in the Water Flea Daphnia and its Impact: An Evolving Metacommunity Approach, Integrative and Comparative Biology, Volume 51, Issue 5, November 2011, Pages 703–718, https://doi.org/10.1093/icb/icr027
Close - Share Icon Share
Abstract
Genetic adaptation to temperature change can impact responses of populations and communities to global warming. Here we integrate previously published results on experimental evolution trials with follow-up experiments involving the water flea Daphnia as a model system. Our research shows (1) the capacity of natural populations of this species to genetically adapt to changes in temperature in a time span of months to years, (2) the context-dependence of these genetic changes, emphasizing the role of ecology and community composition on evolutionary responses to climatic change, and (3) the impact of micro-evolutionary changes on immigration success of preadapted genotypes. Our study involves (1) experimental evolution trials in the absence and presence of the community of competitors, predators, and parasites, (2) life-table and competition experiments to assess the fitness consequences of micro-evolution, and (3) competition experiments with putative immigrant genotypes. We use these observations as building blocks of an evolving metacommunity to understand biological responses to climatic change. This approach integrates both local and regional responses at both the population and community levels. Finally, we provide an outline of current gaps in knowledge and suggest fruitful avenues for future research.
Introduction
Increasing evidence shows a profound impact of global warming on the ecology and distribution of a wide variety of species (Parmesan 2006; Visser 2008; Heino et al. 2009). The selection pressures associated with global warming may result in the development of strategies for thermal resistance in local populations, either by phenotypic plasticity or by evolutionary responses (Parmesan 2006; Visser 2008). Most studies on thermal adaptation mainly focused on environmentally induced plastic responses (Angilletta 2009). As a result, thermal micro-evolution is often ignored when making predictions about the impact of global warming on the survival of populations, the distribution of organisms and/or the composition of communities (Pearson and Dawson 2003).
Ecological and evolutionary processes were until recently generally thought to occur on different time-scales. However, there is strong evidence for rapid and adaptive evolutionary responses with the potential to alter ecological processes (e.g., Hairston et al. 2005; Thompson 2005; Carroll et al. 2007; Fussmann et al. 2007; Urban et al. 2008). There is an increasing number of reports of genetically different lineages or populations of a given species influencing ecological interactions with other species (e.g., Yoshida et al. 2003; De Meester et al. 2007; Harmon LJ et al. 2009). For instance, Jones et al. (2009) have shown that increased evolutionary potential affects community dynamics in a model with multiple predator and prey genotypes. These studies underscore that the occurrence of genetically different strains, and thus micro-evolution, may strongly impact ecological dynamics. The analysis of rapid evolution as an ecological process has the potential for making evolutionary ecology one of the central topics of applied biological sciences (Thompson 1998). This is especially important because the evolving metacommunity framework is very valuable for studying the impact of large-scale, strong environmental changes, such as climatic change, pollution, eutrophication, invasive exotic species, and fragmentation of habitats. The evolving metacommunity framework integrates community ecology, evolution, and regional aspects (dispersal and gene flow, metacommunity structure; Urban et al. 2008).
In an effort to evaluate in a focal species the impact of micro-evolutionary processes on the responses to changes in temperature at both the population and community level, we initiated a research program using large-bodied cladocerans, and the water flea Daphnia magna in particular, as a model system. Being strong competitors, large zooplankters such as Daphnia can be considered keystone species in freshwater ecosystems (Lampert 1987). Owing to this central position in the food web, micro-evolutionary responses within Daphnia may potentially impact the responses of communities to environmental change.
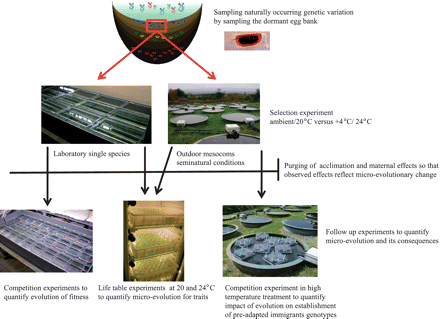
Starting from a representative sample of a natural population of D. magna, our general approach involved exposing the resulting experimental populations to different thermal regimes in experimental evolutionary set-ups, and quantifying the resulting micro-evolutionary responses using life-table experiments as well as competition trials assessing fitness (Fig. 1). As we used different experimental settings, both in highly simplified laboratory set-ups (Van Doorslaer et al. 2009a) as well as in semi-natural outdoor mesocosms (Van Doorslaer et al. 2010), our data also shed light on the impact of the ecological setting on the responses to changes in temperature. Finally, we aimed at explicitly investigating one aspect of ecological consequences of the observed micro-evolutionary responses by quantifying the impact of the genetic changes on the success of immigrant genotypes to become established (Van Doorslaer et al. 2009b). Our key observations are that (1) a change in temperature can induce rapid evolutionary shifts, (2) the nature of these responses is impacted by ecological conditions, and (3) the micro-evolutionary responses impact ecological interactions. In the following paragraphs, we provide a short overview of these observations and put them in a larger context, focusing on the usefulness of an evolving metacommunity framework for our understanding of biological responses to climatic change. We then highlight some important avenues for future research in a section on perspectives, focusing on gaps in knowledge as well as on the promises of “resurrection ecology” and paleogenomics.
Overview of overall design of research on thermal adaptation in the water flea Daphnia magna. The selection experiment in the laboratory and its associated follow-up experiments are presented by Van Doorslaer et al. (2009a). The selection experiment in the mesocosms and the associated life-table experiment are presented by Van Doorslaer et al. (2010). The follow-up study involving competition experiments with preadapted genotypes is presented by Van Doorslaer et al. (2009b).
Rapid thermal micro-evolution
Experimental set-up and main results
We carried out two experimental trials to quantify the capacity of D. magna to genetically adapt to a change in temperature regime (Van Doorslaer et al. 2009a, 2010; Fig. 1 and Table 1). In both cases, we used the dormant egg bank of a natural D. magna population as starting material. More specifically, we took an extensive sample of the dormant egg bank (3600 dormant eggs) of the D. magna population inhabiting Brown Moss (Shropshire, UK) and hatched them in the laboratory. Hatching dormant egg banks yield representative samples of genetic diversity in natural populations, as in nature Daphnia populations are re-established after winter by hatching from the dormant egg banks in the superficial layers of the sediments. We thus obtained representative samples of the resident population inhabiting Brown Moss as starting material for our experiments. By using animals derived from one population, we quantified evolutionary potential of a resident natural population to respond to changes in temperature.
Overview of similarities and differences among the laboratory (Van Doorslaer et al. 2009a) and the mesocosm experiment (Van Doorslaer et al. 2010) used to study thermal adaptation in Daphnia magna using the approach of experimental evolution
| . | Aquarium . | Mesocosm . |
|---|---|---|
| Populations | ||
| Source population | Brown Moss (UK) | Brown Moss (UK) |
| Number of clones | 240 clones/unit | 150 clones/unit |
| Selection experiment | ||
| Size of units | 10 L | 3000 L |
| Temperature regimes | Constant | Varying naturally (outdoor) |
| Culling | 20°C and 24°C | Ambient and +4°C |
| Yes (50% every 15 days; 10% very 3 days) | No | |
| Community of competitors | Absent | Present |
| Parasites | Absent | Present |
| Predators | Absent | Invertebrate predators |
| Macrophytes | Absent | Present |
| Duration of selection | 3 months | 6 months |
| Responses | ||
| Increase in fitness (competition experiments) | Yes | Not quantified |
| Performancea r | Yesb | No |
| Age at release of 1st clutch | No | No |
| No. of 1st clutch offspring | Yesc | No |
| Size at maturity | No | Yesd |
| . | Aquarium . | Mesocosm . |
|---|---|---|
| Populations | ||
| Source population | Brown Moss (UK) | Brown Moss (UK) |
| Number of clones | 240 clones/unit | 150 clones/unit |
| Selection experiment | ||
| Size of units | 10 L | 3000 L |
| Temperature regimes | Constant | Varying naturally (outdoor) |
| Culling | 20°C and 24°C | Ambient and +4°C |
| Yes (50% every 15 days; 10% very 3 days) | No | |
| Community of competitors | Absent | Present |
| Parasites | Absent | Present |
| Predators | Absent | Invertebrate predators |
| Macrophytes | Absent | Present |
| Duration of selection | 3 months | 6 months |
| Responses | ||
| Increase in fitness (competition experiments) | Yes | Not quantified |
| Performancea r | Yesb | No |
| Age at release of 1st clutch | No | No |
| No. of 1st clutch offspring | Yesc | No |
| Size at maturity | No | Yesd |
Note. The experiments differed in several aspects that may have influenced responses to selection. This makes a direct comparison of the micro-evolutionary responses in the two designs less straightforward. Yet, the experiments do share three important features: they start with material from the same natural population (the number of clones is different, but in both cases high enough so that they can be representative samples of resident genetic diversity), selection involved a near-optimal versus a +4°C temperature regime, and the experiment lasted a few months.
aPerformance is quantified as intrinsic rate of population increase but without taking mortality into account.
bIncrease in performance after selection in the 24°C regime compared to after selection in the 20°C regime, in the 50% culling regime only.
cIncrease in number of offspring of 1st clutch after selection in the 24°C regime compared to selection in the 20°C regime, in the 50% culling regime only.
dReduction of phenotypic plasticity (smaller reduction in size at maturity at higher temperature) of animals exposed to the high temperature regime.
Overview of similarities and differences among the laboratory (Van Doorslaer et al. 2009a) and the mesocosm experiment (Van Doorslaer et al. 2010) used to study thermal adaptation in Daphnia magna using the approach of experimental evolution
| . | Aquarium . | Mesocosm . |
|---|---|---|
| Populations | ||
| Source population | Brown Moss (UK) | Brown Moss (UK) |
| Number of clones | 240 clones/unit | 150 clones/unit |
| Selection experiment | ||
| Size of units | 10 L | 3000 L |
| Temperature regimes | Constant | Varying naturally (outdoor) |
| Culling | 20°C and 24°C | Ambient and +4°C |
| Yes (50% every 15 days; 10% very 3 days) | No | |
| Community of competitors | Absent | Present |
| Parasites | Absent | Present |
| Predators | Absent | Invertebrate predators |
| Macrophytes | Absent | Present |
| Duration of selection | 3 months | 6 months |
| Responses | ||
| Increase in fitness (competition experiments) | Yes | Not quantified |
| Performancea r | Yesb | No |
| Age at release of 1st clutch | No | No |
| No. of 1st clutch offspring | Yesc | No |
| Size at maturity | No | Yesd |
| . | Aquarium . | Mesocosm . |
|---|---|---|
| Populations | ||
| Source population | Brown Moss (UK) | Brown Moss (UK) |
| Number of clones | 240 clones/unit | 150 clones/unit |
| Selection experiment | ||
| Size of units | 10 L | 3000 L |
| Temperature regimes | Constant | Varying naturally (outdoor) |
| Culling | 20°C and 24°C | Ambient and +4°C |
| Yes (50% every 15 days; 10% very 3 days) | No | |
| Community of competitors | Absent | Present |
| Parasites | Absent | Present |
| Predators | Absent | Invertebrate predators |
| Macrophytes | Absent | Present |
| Duration of selection | 3 months | 6 months |
| Responses | ||
| Increase in fitness (competition experiments) | Yes | Not quantified |
| Performancea r | Yesb | No |
| Age at release of 1st clutch | No | No |
| No. of 1st clutch offspring | Yesc | No |
| Size at maturity | No | Yesd |
Note. The experiments differed in several aspects that may have influenced responses to selection. This makes a direct comparison of the micro-evolutionary responses in the two designs less straightforward. Yet, the experiments do share three important features: they start with material from the same natural population (the number of clones is different, but in both cases high enough so that they can be representative samples of resident genetic diversity), selection involved a near-optimal versus a +4°C temperature regime, and the experiment lasted a few months.
aPerformance is quantified as intrinsic rate of population increase but without taking mortality into account.
bIncrease in performance after selection in the 24°C regime compared to after selection in the 20°C regime, in the 50% culling regime only.
cIncrease in number of offspring of 1st clutch after selection in the 24°C regime compared to selection in the 20°C regime, in the 50% culling regime only.
dReduction of phenotypic plasticity (smaller reduction in size at maturity at higher temperature) of animals exposed to the high temperature regime.
Using mixtures of clones derived from the same population (Brown Moss), we carried out two selection experiments that were designed to quantify responses to a change in temperature under two widely differing ecological settings. In the first experiment, carried out in 10 L aquaria in the laboratory, we exposed the Daphnia to two constant temperature regimes (20 and 24°C) as pure cultures, i.e., in the absence of competitors, predators and parasites (Van Doorslaer et al. 2009a). In the second experiment, we carried out a selection experiment in 3000 L mesocosms that were inoculated with natural communities in addition to the D. magna clones from Brown Moss. The mesocosms contained a rich community of a large number of interspecific competitors, invertebrate predators, and parasites (Feuchtmayr et al. 2009; Jeppesen et al. 2010). For this study, we capitalized on a large-scale ecological experiment designed to study the combined impact of nutrient loading, temperature and predation on replicate pond ecosystems (Feuchtmayr et al. 2009). The experiments (laboratory and mesocosm) differed in several aspects (Table 1; more details on methods in Van Doorslaer et al. 2009a, 2010), but shared two important aspects: (1) the starting population, and (2) the same large number of clones used to start the experiment, representative of the genetic diversity in the resident population. They also shared the same temperature regimes in which one was near-optimal or ambient and the other involved a rise in temperature of 4°C (inspired by global-warming scenarios; IPCC 2007); in both experiments, the selection regimes lasted for a few months (3 months in the aquarium; 6 months in the mesocosm).
When cultured in the laboratory in the absence of interspecific competitors, predators, and parasites, populations of D. magna showed rapid differential micro-evolutionary responses to different temperature regimes (20°C versus 24°C) in one of the culling regimes (removal of 50% of the animals every 15 days; there was no response in the regime with 10% culling every 3 days; (Van Doorslaer et al. 2009a) (Table 1). Already after 3 months of selection, populations selected at the highest temperature (24°C) performed better in terms of their intrinsic population growth rate both at a test temperature of 24°C as well as at the test temperature of 20°C. Our results also provide indications of adaptive evolution of thermal plasticity under experimental warming.
In our experiments using mesocosms, in which D. magna co-existed with a community of competitors, predators and parasites under semi-natural conditions, we observed significant genetic changes in size at maturity within the short period (6 months) during which the experiment was conducted. More specifically, body size of the Daphnia selected at the higher temperature regime (ambient + 4°C) showed a less strong response to temperature than did animals selected at ambient temperature. Daphnia become smaller at higher temperatures, but the response is less strong in animals previously kept in the mesocosms exposed to a higher temperature regime than in animals from the mesocosms at ambient temperature (Van Doorslaer et al. 2010). The same response was also observed for Daphnia pulex, another Daphnia species that occurred in these mesocosms (Van Doorslaer et al. 2010). In another study involving the large-bodied cladoceran Simocephalus vetulus, we observed an increased thermal tolerance (Van Doorslaer et al. 2007).
In each of these studies, a rapid genetic change was observed upon exposure to a different temperature regime. In addition, these micro-evolutionary changes were adaptive. This is clear for Simocephalus which showed increased survival (Van Doorslaer et al. 2007) and for D. magna which showed increased population growth in the aquarium experiment (Van Doorslaer et al. 2009a). The adaptive nature of the response in size at maturity observed in the mesocosm experiment was less straightforward to assess (Van Doorslaer et al. 2010), but we could show in follow-up experiments that adaptive genetic changes occurred (Van Doorslaer et al. 2009b).
Methodological considerations
We observed clear shifts in ecologically relevant traits in response to the temperature treatments in our selection experiments. Yet, there are methodological limitations that may interfere with too bold an interpretation of our results in the context of global change. We here briefly discuss strengths and weaknesses of our approach.
Temperature regimes: timing and amplitude
We observed significant evolutionary responses to temperature in all our selection experiments. Yet, one may argue that our temperature regimes were quite artificial. Although inspired by predictions of widely supported models (IPCC 2007), we indeed applied a sledge hammer approach by immediately exposing the selected populations to a temperature increase that is in reality predicted to rather gradually occur over a period of 100 years. A gradual change in selection pressures may result in a different evolutionary response than would a sudden change (e.g., Collins et al. 2007; Gienapp et al. 2008). Although the comment is without doubt valid, our experimental approach focuses on the evolutionary potential to respond to the predicted changes rather than on the precise trajectory of evolutionary change. By applying a much stronger selection gradient than is expected to occur in nature and monitoring genetic responses over very short time spans, we tested the limits of the studied populations to respond to a temperature change by micro-evolution. Although the details of the responses we observed should not be interpreted too boldly, our results do indicate that local populations have the capacity to show micro-evolutionary adjustments to changes in temperature. At the least, this shows that there is relevant evolutionary potential, which is likely to influence the ecological responses of local populations. Finally, the shifts in temperature applied in our experiments are realistic in the long run, as realistic scenarios of climatic change predict more extreme climatic events in the future. The amplitude of the temperature change (+4°C) and the time frame over which we monitored responses to selection (several months up to an year) are very relevant in the context of intra-annual or inter-annual variation, and thus predicting responses to years with, for instance, an exceptionally warm spring or summer. Our experimental set-up is a simplification of what happens in wild populations in another perspective. We did not analyze responses to an increased variation in temperature. Indeed, not only a step-wise versus a steady temperature increase may affect the outcome of evolutionary responses (e.g., Collins et al. 2007), but also constant versus fluctuating temperature regimes potentially may be important in driving micro-evolution (e.g., Kingsolver et al. 2009). Both, the mean temperature experienced by organisms in their habitat as well as the extent of fluctuations (frequency and amplitude) in habitat temperature may be important during the life cycles of organisms. A study by Blanford et al. (2003) shows that variation in environmental temperature can be seen as a mediating factor in the expression of genotypic variation on which selection can act. There is a need for more experiments that explicitly test for micro-evolutionary responses to increased variation in temperature (e.g., Leroi et al. 1994).
Artificial settings
More generally, it is clear that our experiments represent simplifications of natural conditions, which may interact with the evolutionary responses we observe. This is true for any experimental study of evolution, and cautions against an over-interpretation of the results. Yet again, our experiments in the first place show that there is important evolutionary potential for responses to changes in temperature. An important aspect that adds to the relevance of our results is that we carried out different experiments under widely different environmental conditions, and that the key observation of a rapid evolutionary response to the temperature change was observed under all these settings. Our experiments thus point to the generality of the capacity of local populations to respond genetically to a sudden change in temperature change over a short interval of time. They also point to the fact that the specifics of those responses are dependent on the ecological settings (see below).
Genetic versus maternal effects
Our experimental procedure involved isolating individuals from selection units and culturing these as separate clonal lineages for several generations before carrying out life-table experiments at different temperatures. As a result, our experiments did not confound maternal with genetic influences, and the genetic differences among populations exposed to different temperature regimes that we report reflect micro-evolutionary responses. While we carefully obviated interference from maternal effects, this does not imply that maternal effects may not be important in shaping responses to global warming in nature (e.g., see Visser 2008). Several studies have shown that maternal effects may be of evolutionary significance as they provide a mechanism for adaptive transgenerational phenotypic plasticity (e.g., reviewed in Mousseau and Fox 1998) and may dramatically enhance rates of evolutionary responses to selection (reviewed by Mousseau et al. 2009).
Evolution as changes in relative abundance of clones
Our experiments mimic the natural situation in two important ways: (1) we inoculated our experimental populations using dormant eggs from the superficial layers of the sediments; this is exactly what happens in nature at the beginning of the growing season, and (2) we initially inoculated the selection units with genetically diverse populations, and then monitored the genetic changes over time during the selection experiment. This mimics what happens in nature, where genetically diverse populations are exposed to clonal and environmental selection after establishment. In addition, the initial populations used in the experiments were genetically standardized and consisted of a given number (more than 100) of genetically distinct clones that were replicated over experimental units, making comparisons across experiments possible. All, or at least most, genetic responses observed in our experiments reflect changes in the relative abundance of the inoculated clones rather than the genetic variation generated by mutations typically studied in experimental evolution trials with bacterial communities (e.g., Remold and Lenski 2001; Rifkin et al. 2005) or generated by sexual recombination (e.g., Tessier et al. 1992; Goddard et al. 2005). As evolution is defined as a change in frequencies of alleles (e.g., Ridley 2003; Freeman and Herron 2007), the observed changes in the relative frequency of clones clearly reflects micro-evolutionary responses at the population level. Significant changes in clonal diversity and heritability of life-history traits have been observed within a short time-span (months) in natural Daphnia populations (e.g., Lynch 1984; Tessier et al. 1992). We can exclude that such changes were induced by sexual reproduction events, because such events would have resulted in a different genotypic composition than the initial population. What we observe is only a change in the relative abundance of genotypes inoculated in the initial population. Thus, by definition, the observed changes in relative frequency of clones reflect evolution and may reflect an essential part of the micro-evolutionary responses of any species that combines asexual and sexual reproduction.
It would be very useful to quantify the dynamics of the evolutionary responses over several cycles of reproduction, i.e., involving both parthenogenetic and sexual reproduction. It can be expected that sexual reproduction will result in genetic slippage of the mean genotypic value in a direction contrary to that resulting from selection (Lynch and Deng 1994) and thus, temporarily lower fitness. On the other hand, sexual reproduction generates novel genetic variation, which may increase the response to selection during the subsequent phase of parthenogenetic reproduction (e.g., Tessier et al. 1992).
Importantly, in our design we started from samples of naturally occurring genetic variation, as we started from dormant egg banks. Natural populations of the species studied thus, harbor enough genetic variation at the start of the growing season to show these rapid genetic responses. We did not mix genotypes from different latitudes or other temperature-related gradients to artificially increase genetic variation for temperature-associated traits. Our results show that significant genetic changes in ecologically relevant traits can be observed after only a few months of thermal selection in large-bodied cladocerans, which are considered keystone species in standing inland waters. Evolutionary responses thus occur at ecological time-scales and may interfere with ecological processes (Hairston et al. 2005; Pelletier et al. 2009). We discuss this in more detail below.
Importance of ecological context
Evolutionary responses take place in an ecological setting that typically involves interaction between individuals and the dynamics of changing populations and communities in addition to abiotic environmental conditions. Our results underscore the importance of the ecological context in shaping micro-evolutionary responses. First, the results reported by Van Doorslaer et al. (2009a) show that the laboratory environment itself represents a selection pressure upon the populations. We indeed observed strong differences in life-history traits between populations cultured in the laboratory aquaria and the initial populations, which were hatched from dormant eggs and represent a random sample of genotypes from the field. This suggests that the selective environment in the laboratory experiment was different than the one in the field, where the populations face multiple selection forces including biotic interactions such as predation, interspecific competition and parasites. Secondly, we observed that strong fluctuations in population size imposed by one of our culling regimes (50% every 15 days instead of 10% every 3 days) and thereby creating recurrent periods during which exponential growth is possible, enhanced the rate of micro-evolution. This indicates that population dynamics may represent an important ecological determinant of evolution, as they establish the relative importance of growth rate and competitive strength (de Roos and Persson 2003; Nelson et al. 2005). Third, the micro-evolutionary responses obtained in the laboratory (Van Doorslaer et al. 2009a) and mesocosms (Van Doorslaer et al. 2010) were quite different (Table 1). This suggests that the ecological setting (amongst others, single-species isolated cultures versus community-embedded populations) is important in driving micro-evolutionary change and in determining which traits adapt to a change in temperature. In their review of experimental evolution studies, Reznick and Ghalambor (2005) similarly stress that controled laboratory conditions yield quite different outcomes for stress responses of organisms compared to field studies in which species are subjected to a wide variety of selective forces, including trade-offs between different stressors.
Our observation that the broader ecological context strongly influences the resulting micro-evolutionary responses is important, as it stresses the complexity of predicting evolutionary responses, and cautions against too bold an extrapolation of laboratory experiments that are carried out in an over-simplified environment. At the same time, this warning also applies to our own mesocosm experiment, because the mesocosms, even though more complex, can hardly be considered true replicas of real lakes. As reviewed by Angilletta (2009), both, laboratory and mesocosm experimental designs have their advantages and disadvantages. Selection experiments in the laboratory, which often only include intraspecific interactions, directly link environmental temperatures to the evolution of phenotypes. Any difference between the phenotypes of experimental and control lines can be attributed to thermal adaptation. Laboratory experiments are thus well-suited to test the potential for evolutionary responses to temperature changes, but they are a weak imitation of natural conditions. Field experiments or experiments under semi-natural conditions in outdoor mesocosms may reveal novel patterns of thermal adaptation because natural selection depends on the interaction (including trade-offs) of temperature and other environmental and biotic factors. This increases environmental realism to an important degree, but clearly complicates straightforward interpretations of the results. The micro-evolutionary responses that we observe in our mesocosm experiment may be caused by direct selective effects of temperature as well as by indirect temperature-mediated effects via changes in predation pressure, competitive interactions, availability of food, and/or prevalence of parasites. It is impossible to disentangle the impact of these different potential causes of genetic changes without performing extensive additional experiments. By carrying out both laboratory and outdoor mesocosm experiments, however, we did cover two important and widely different points in the spectrum of ecological complexity in our assessment of micro-evolutionary responses to temperature change.
Ecological consequences of evolution
Given that the micro-evolutionary changes we observed in our experiments occur in a short span of time, they have the potential to influence ecological processes (e.g., Hairston et al. 2005; Urban et al. 2008). In a follow-up study to the mesocosm experiment (Van Doorslaer et al. 2009b), we performed an experiment to test this for one specific interaction. More particularly, we quantified to what degree genetic adaptation may increase the capacity of a resident population to reduce success of immigrant genotypes to establish. As we wanted to mimic a scenario relevant under climatic change, we carried out our experiments at increased temperature (ambient + 4°C) and used genotypes from a warmer region (southern France) as immigrants to compete with nonadapted and warm-adapted UK residents (cfr. Brown Moss population). The results were striking. First, the southern genotypes had a very strong fitness advantage over the UK residents in the competition trials at elevated temperature (ambient + 4°C; Van Doorslaer et al. 2009b). Second, the southern genotypes also had higher fitness at elevated temperatures than did the resident populations that were allowed to adapt genetically to the increased temperature regime during 1.3 years. However, their fitness advantage was strongly reduced compared to their fitness advantage when confronted by nonwarm-adapted populations (Van Doorslaer et al. 2009b). These observations show that the observed micro-evolutionary responses are relevant with respect to competitive interactions of the local population with immigrant genotypes.
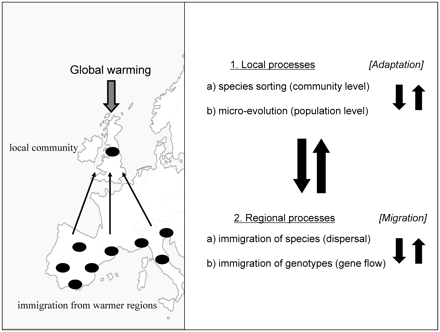
We calculated that under realistic circumstances, the increased competitive strength of warm-adapted residents would result in a more than doubling of the time for the immigrant genotypes to become dominant in the local community, and that the number of immigrants would have to be 104–105 times higher to result in the same speed at which half of the population would consist of immigrants when the immigrants have to compete with warm-adapted rather than with nonwarm-adapted resident populations (Van Doorslaer et al. 2009b). This is a striking difference to result from only 1.3 years of micro-evolutionary change. Unfortunately, the mesocosm selection experiment was terminated in the second year (Feuchtmayr et al. 2009), as it would have been instructive to monitor further micro-evolutionary changes and to determine whether and when local residents and preadapted immigrant genotypes could become equally adapted to the high temperature regime. Two expected impacts of genetic tracking of environmental change on community responses to climatic change are (1) that species composition of local communities will change less in the presence than in the absence of micro-evolutionary adaptation, and (2) that responses of communities to global warming will be more localized in the presence than in the absence of evolution. Environmental changes are expected to impact the relative fitness of different species in a community. However, if local populations genetically track environmental changes in a way that their fitness relations remain unchanged, this may translate in highly reduced changes in species composition compared to scenarios without evolution (Fig. 2). In previous mesocosm studies investigating temperature-mediated ecological effects related to global warming, temperature often only had a marginal effect on community composition (e.g., McKee et al. 2002; Moss et al. 2003). In principle, part of this absence of a shift in species composition may be mediated by shifts in genotype composition within species, i.e., evolution. The response of a local community to global warming may be mediated by local and regional processes (Fig. 2), as one may expect changes both in the relative abundance of resident species in the community, as well as immigration of new species from the regional species pool. Specifically in the case of global warming, immigration may be important. In the northern hemisphere, organisms may be successful when migrating northwards from southern locations, as they are preadapted to a warmer climate. Our results, however, show that local genetic tracking of temperature change in resident populations may reduce establishment success of immigrant genotypes (Van Doorslaer et al. 2009b). Further experiments are needed to test whether this also applies to immigration of different species. In an earlier experiment, which did not focus on temperature selection, it was shown that differences in the genotypic composition of D. magna may indeed impact the success of species of the regional species pool to become established (De Meester et al. 2007). These combined results indicate that it is not unlikely that local adaptation to temperature change may impact the success of species in immigrating from other regions. If so, evolution would have as a net effect, a reduction of the importance of regional processes (immigration) in the response of local communities to global warming.
A schematic representation of the evolving metacommunity approach to study biological responses to climatic change. When a local community is exposed to climatic change, it may respond locally both by changes in species composition (species sorting) as well as by genetic adaptation of local populations that build up the community (micro-evolutionary change). Both responses may interact with each other: If local genetic adaptation efficiently tracks environmental change, it may reduce the impact of species sorting, whereas rapid replacement of species may not allow time for specific species to respond by evolutionary change. In addition to the local responses, there may also be a regional impact, through immigration of novel genotypes and/or species from other populations. These may be preadapted to the local environmental conditions that result from environmental change. In a climate-change scenario, this would involve immigration of genotypes or species from warmer regions inhabited by populations and species that are preadapted to higher temperatures. Importantly, local and regional processes interact, as efficient tracking of climatic change by local processes may reduce success of immigrants in becoming established, whereas rapid replacement of resident genotypes and species by preadapted immigrants may not allow time for local species sorting or micro-evolution. This interplay between local and regional processes and between micro-evolution and species sorting is the key focus of an evolving metacommunity perspective.
Although important life-history traits clearly evolved during experimental warming, one should not conclude from our results that micro-evolutionary responses will “solve” the problems associated with climatic change. No doubt, rapid genetic tracking of global warming may reduce extinction rates of local populations and the impact of migration, but there are several reasons why one should be careful not to over-interpret our observations. First, there is the observation that global warming already has had a clear impact during the past decades, including replacements of species, mismatches in interactions between species, local extinctions, and invasion of warm-adapted species (e.g., Thomas et al. 2004; Hickling et al. 2006; Durant et al. 2007; van der Wal et al. 2008). Second, one should not expect perfect genetic tracking of changes in a way that the interactions of species with their abiotic environment or with other species would not change: (1) species differ in their capacity to evolve because of differences in generation time, genetic variation, and ecological, genetic, and phylogenetic constraints. This will change interactions among species, including interactions with food, competitors, predators, and parasites, and (2) genetic adaptation to novel conditions may entail costs, both in terms of reduced energy or reduced evolutionary potential to respond to other stressors (e.g., Eranen et al. 2009). In short, temperature is not the only environmental selective force that organisms encounter in their habitat, and complex biotic interactions and combined effects of multiple abiotic stressors both are expected to complicate responses to global warming.
Overall, our results suggest that genetic adaptation may occur rapidly and is likely to have far-reaching ecological consequences (see also Jones et al. 2009; Pelletier et al. 2009). From the previous paragraph, it is clear that micro-evolution will not necessarily mitigate the impact of global warming, but rather change the impact. These changes may include an alteration in the relative importance of biotic interactions and different stressors, or an increased capacity of resident populations to respond by internal dynamics and thus reduce the impact of immigration from the regional genotype and species pool. From our results, it is clear that it is important to include evolutionary responses in predictions of changes in community composition in response to global warming. In most research and models predicting the potential geographic distribution of species under current and future climatic scenarios (so-called “bioclimate envelopes”), evolutionary responses are ignored (e.g., Pearson and Dawson 2003). This is likely to lead to wrong predictions (e.g., Pearson et al. 2006; Skelly et al. 2007). Given the rates of evolutionary change, we observe in our experiments, the degree to which these predictions are wrong could be problematic, not necessarily because the models would overestimate the impact of climatic change, but rather because the real impact may be of a different nature.
Future perspectives
Our results provide evidence for rapid and adaptive micro-evolutionary responses to increases in temperature, and show that these genetic changes are likely to impact ecological responses to global warming. In the following paragraphs, we present some ideas for future research that are inspired by our results as well as by the methodological limitations of the work presented here. These future perspectives refer to the need to broaden our knowledge of evolutionary responses to climatic change by including a wider range of organisms, systems, and conditions, the possibilities offered by new approaches and techniques, and the need to further study ecological implications of micro-evolutionary responses to climatic change.
Broadening of the scope
Beyond Daphnia of temperate shallow lakes
Our study focused on micro-evolutionary responses to temperature change in large cladocerans such as D. magna (Van Doorslaer et al. 2009a, 2009b) and S. vetulus (Van Doorslaer et al. 2007). Although these species may dominate zooplankton communities in shallow standing waters in temperate regions and may play a key role in the ecology of these systems, they are typically only important in temperate climatic zones and in shallow ponds and lakes. There is a need for studies on micro-evolutionary responses to climatic change of zooplankton in deep lakes and in the marine realm. The responses of deep-lake biota to increasing temperature may be different from those of shallow-water biota, as deep lakes are thermally stratified and thus provide additional opportunities for organisms to respond to changes in thermal conditions by changing their vertical-migration behavior. There is also a need to study responses to global warming in warm semi-arid regions and in tropical systems. Temperature change is expected to be of less amplitude in the tropics, but environmental temperatures are already high and may be close to the thermal tolerance limits of several species (e.g., for tropical terrestrial species, see Deutsch et al. 2008; Huey et al. 2009). A study by Stillman (2003) shows that marine Porcelain crab species that have evolved the greatest tolerance to high temperatures have done so at the expense of their capacitiy for acclimation, and these species will be the most susceptible to increases in microhabitat temperatures. Also, large lakes, rivers, and marine systems differ widely in dimensions, and north–south-oriented rivers and marine systems (e.g., Beaugrand et al. 2002) may provide more opportunities for migration in response to climatic change along a habitat continuum than do the ponds and lakes inhabited by our model species D. magna, and this may strongly modulate the observed responses.
Realistic scenarios of change
As already mentioned, we have made some pragmatic decisions concerning the temperature regimes that we used in our experiments. Although our choice to impose a sudden temperature increase of +4°C can be viewed as an acceptable compromise between realism and feasibility, it is clear that this approach has intrinsic limitations. Two important and feasible additions would be to vary both the speed of temperature change, and to expose populations to treatments with different amplitudes of temperature change. The first experiment may, for practical reasons, not include realistic rates of temperature change such as a change of <1°C in a decade, but would allow quantifying the degree to which different rates of temperature change may impact evolutionary responses of populations. The second experiment would test the impact of an important expected characteristic of future climate: increase in extreme temperatures.
The broader community and trophic cascades
Zooplankton communities interact with both, their prey populations and with a wide range of predators, and these interactions are likely to be strongly impacted by global warming (Tylianakis 2009; Gilman et al. 2010). It has been shown that increased temperature has the potential for destabilizing planktonic food webs (Beisner et al. 1997; Strecker et al. 2004; Wagner and Benndorf 2007). Although our mescocosm experiments, by providing semi-natural conditions, took this complexity caused by trophic interactions into account to some extent, there is a need for studies that also quantify genetic responses to climatic change by phytoplankton, macro-invertebrates and fish. It has recently been shown that different ecotypes of three-spine sticklebacks may have a different impact on ecosystem characteristics (Harmon LJ et al. 2009), indicating that climate-driven evolutionary change in predators may cascade down along the food web (see also Harmon JP et al. 2009). In addition, there is good reason to expect bottom–up effects of climate-driven evolutionary responses in phytoplankton. Yoshida et al. (2003) showed that genetic diversity in algae may strongly impact predator–prey dynamics in a zooplankton–alga system, and the short generation time of phytoplankton may provide ample scope for rapid evolutionary changes (e.g., see Vanormelingen et al. 2009).
Multiple stressors
Global warming is not the sole stressor to which natural populations are exposed to. It is becoming increasingly clear that the vulnerability of freshwater communities to global warming can be exacerbated by other anthropogenically induced large-scale environmental stressors such as eutrophication, metal pollution, habitat destruction, UV irradiance, and introductions of species (reviewed by Bronmark and Hansson 2002; Sokolova and Lannig 2008). In addition, climatic change itself may exacerbate problems of eutrophication and exotic species, amongst others (Kernan et al. 2010). These different anthropogenic stressors may synergistically interact, leading to intensified negative impacts on populations, species, and communities. Several studies reported nonadditive effects of stressors (e.g., Coors and De Meester 2008), while others reported temperature-mediated effects of predation, food concentration, pesticides, metal pollution, and/or parasitism on freshwater communities (e.g., Folt et al. 1999; Giebelhausen and Lampert 2001; Mitchell et al. 2005; Bernot et al. 2006; Heugens et al. 2006; Vale et al. 2008). There is a need for studies that quantify patterns and constraints of micro-evolutionary responses to multiple stressors in the framework of climatic change.
Novel approaches to the study of evolutionary responses to global warming
Resurrection ecology
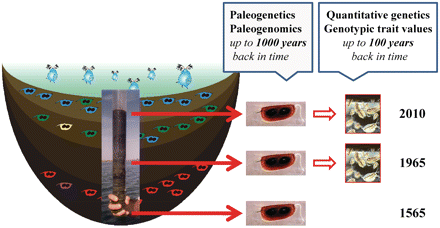
Assessing the impact of global warming under natural conditions generally requires long-term datasets and monitoring. Records of freshwater zooplankton in sediments, however, offer the possibility of extending the timescale of observation, provided that the environmental changes of interest also occurred in the past. Many organisms inhabiting inland waters produce dormant stages when environmental conditions deteriorate. These dormant stages accumulate in the sediment and form dormant egg banks. As sediments in lakes often are layered, these dormant egg banks can be viewed as historical archives harboring valuable information on the history of the habitat (including selective pressures) and the resident populations and communities (reviewed by Jeppesen et al. 2001; Brendonck and De Meester 2003; Smol and Douglas 2007; Hairston and De Meester 2008; Fig. 3). The “resurrection ecology” approach involves hatching of dormant eggs of different age to compare populations from different time periods. The procedure for comparing these populations can be similar to the one we used to compare populations in our experimental evolution trials. By comparing representative samples from different times, it is possible to document rates and patterns of evolution (e.g., Hairston et al. 1999; Cousyn et al. 2001; Decaestecker et al. 2007). Given that there is a clear signal of global warming during the course of the past decades, this approach can also be applied to reconstruct responses to climatic change (Angeler 2007; Franks et al. 2007, 2008).
In addition to the experimental evolution approach outlined in the present work, in which one starts with a representative sample of the recent dormant egg bank of natural zooplankton populations, one can also sample different sedimentary layers that represent an archive of the history of the resident population to study responses to climatic change in the recent past. The layered dormant egg banks can be used in a “resurrection ecology” approach, in which one hatches dormant eggs from different time periods and compares their traits (e.g., Cousyn et al. 2001). In addition, it is also possible to apply a paleogenomics approach, using variation in genes underlying traits to reconstruct micro-evolutionary changes through time. The main advantage of the paleogenomics approach is that it is not dependent on the capacity to hatch dormant eggs, so that the time frame over which micro-evolutionary responses can be studied can be extended substantially. We here, list 1565 as a date of interest as it represents a period of relatively low temperatures (“little ice age” in the Middle Ages). The 1960s are a decade characterized by relatively low temperatures, whereas the recent decade is characterized by high average temperatures globally.
Genomics
In our study, we quantified responses in ecologically relevant traits through a quantitative genetics approach. The promise of ecological and functional genomics in studies of climatic change is high, as genomic tools may allow one to directly assess selection at the gene level and link genetic variation at the DNA level directly to traits’ values (e.g., reviewed in Vasemagi and Primmer 2005; Hoffmann and Daborn 2007). The genetic basis of adaptive shifts has been identified in several studies from a combination of candidate gene studies, genome scans, and expression studies (e.g., Hanski and Saccheri 2006; Pool and Aquadro 2007; reviewed in Hoffmann and Willi 2008). Understanding the genetic underpinning of the adaptation of organisms to changing environments is of pivotal importance in evolutionary ecology. However, it is often a challenging task because it is nearly impossible to unambiguously attribute selective forces within multidimensional selection regimes. Daphnia offers unique opportunities for the study of adaptation and environmental genomics for several reasons: (1) a key asset of Daphnia is the production of dormant stages that accumulate in layered egg banks and represent a valuable resource for the study of evolution in natural populations using the approaches of resurrection ecology and paleogenetics (Hairston et al. 1999; Decaestecker et al. 2007) (Fig. 3). Dormant stages represent the history of evolution during environmental changes and can be used to reconstruct the genomics of evolutionary responses over long periods of time, (2) the rapid progress of techniques in genomics, in particular DNA sequencing, opens new perspectives for nonmodel genetic species. More importantly, genomic tools for Daphnia, including the full genome sequence, are currently being generated at a very fast rate, thanks to the coordinated effort of the Daphnia Genomics Consortium (www.wfleabase.org), and (3) the short generation time of Daphnia allows an experimental evolution approach, pivotal to verify the signature of adaptation in the wild when reverse genetics is not available (this is typical of nonmodel genetic species). The rapid progress of genomics and the key assets of Daphnia open new enthralling perspectives for answering questions of central interest in ecological genetics. How many and what genes are responsible for adaptive responses in the wild? Does adaptation following an environmental change arise more frequently from standing genetic variation or from new mutations? What type(s) of genetic variation (polymorphism in coding regions, regulatory changes, gene duplications, inversions) is responsible for adaptation? Is adaptation the result of many genes of small effect or of few genes of large effect? These questions have always intrigued evolutionary biologists and for years they have tried to answer them through empirical and theoretical approaches. However, these questions remain only partly addressed because of technical and experimental limitations. The availability of next-generation sequencing technologies associated with a well-known ecological context in species with a central role in natural ecosystems has the potential to revolutionize genomic research and enables us to focus on a large number of outstanding questions that previously could not be addressed effectively.
The broader framework: evolving metacommunities
The responses of biological communities to climatic change may be profoundly impacted by the interactions among responses at the population and the community level, and among local and regional processes (see Fig. 2; evolving metacommunity approach, Urban et al. 2008). At the level of the local community, environmental change may cause both a shift in species composition (species sorting) as well as a selection-mediated genetic change in trait values of resident species (micro-evolution). These two processes may interact with each other, as on the one hand, genetic adaptation of resident species may reduce their replacement by other species, whereas at the other hand a population may be displaced before it had time to genetically adapt to the changing environmental conditions.
These local processes, however, interact with regional processes. The response of a local community may indeed also be modulated by immigration of species from elsewhere (Fig. 2). This may include species from the regional species-pool at the landscape level, but may also involve long-distance dispersal. It has indeed been shown that regional influences may occur at different geographic scales from high rates of dispersal and associated genetic exchange between habitats located only a few meters from each other up to dispersal of organisms over >1000 km (e.g., Frisch et al. 2007). Especially in aquatic organisms producing dormant stages, occasional long-distance dispersal of individuals from other latitudes by migrating birds is possible (Figuerola and Green 2002; Green and Figuerola 2005; Frisch et al. 2007). The long-distance dispersal of organisms from regions with a warmer climate has the potential of being an important driver of ecological responses to climatic change. This regional influence may again involve both responses at the population level (immigration of new genotypes; gene flow) as well as responses at the community level (immigration of new species; dispersal).
Importantly, the local and regional processes as outlined here may interact; a strong local response may reduce the impact of immigration, whereas successful immigration of warm-adapted genotypes or species may shortcut local responses (Fig. 2). However, in addition to preventing local populations from matching their local selective optima, gene flow may also contribute to an increase in genetic variation, thereby increasing the adaptive potential that allows populations to track changing thermal optima under global warming (the swamping and spreading effect of gene flow at the margins of the geographic range is reviewed by Bridle and Vines 2007). It may indeed be that the genetic variance present in a local population will not be sufficient for repeated adaptive adjustments to continuously increasing temperatures, and that preadapted genotypes from warmer regions entering local populations may facilitate a sustained response to thermal selection. Understanding how these various processes interact and shape the responses of local communities to climatic change is of key importance for, amongst others, adjusting the current models predicting changes in species distribution and biodiversity in response to climatic change. Current models predict that many species will expand their geographic range northward, thereby following the shifting temperature zones and resulting in colonization of new habitats (“bioinvasions”) with potential conflicts with native species through altered competitive and/or predatory interactions (Rahel and Olden 2008; Rahel et al. 2008).
Our results indicate that rapid micro-evolution may enhance the capacity of native populations to reduce immigration of southern warm-adapted (invasive) individuals of the same species. So far, however, we did not yet include parallel responses at the community level and the interaction between local genetic and community responses (e.g., see Dangles et al. 2008; le Roux and McGeoch 2008; Schaefer et al. 2008). In general, there is a need to quantify the impact of micro-evolutionary changes on: (1) Species sorting in the local community; it is conceivable that genetic adaptation reduces the degree to which species composition changes in response to environmental change, but so far this has not been quantified; (2) Establishment of immigrant species; similar to the reduction in the success of immigrant genotypes to become established shown by Van Doorslaer et al. (2009b), one may expect that genetic adaptation of local populations to environmental change may reduce immigration rates of species from the regional species-pool; (3) Trophic interactions and structure; micro-evolutionary responses may not only change competitive strength of species in the local community (e.g., Peck et al. 2009), but may also alter predator–prey (Harmon JP et al. 2009) and host–parasite dynamics. Organisms at different trophic levels tend to differ strongly in generation time (cf. rate of adaptation) and dispersal rates. This may yield intriguing dynamics, which are currently, however, very poorly understood; and (4) Ecosystem functions and services; In general, micro-evolutionary responses may impact ecosystem characteristics and functions (e.g., Harmon LJ et al. 2009), and thus also ecosystem services. Although freshwater ecosystems provide a wide variety of valuable goods and services for human societies, awareness of the need to conserve freshwater biodiversity seems limited. Ecosystems’ goods and services represent the benefits human populations derive, directly or indirectly, from ecosystems’ functions (Dudgeon et al. 2006). The variety of benefits provided by freshwater ecosystems include regulation of waste and nutrients; retention of soil; provisioning of water and food (fish); and cultural services such as recreation (e.g., Farber et al. 2006). If micro-evolutionary responses impact the resilience of ecosystems or their capacity to resist change, or other ecosystem functions such as the efficiency of energy transfer and productivity, then this may strongly impact the various ecosystem services mentioned above. Clearly, there is still much to learn, and in our opinion the concept of evolving metacommunities (Urban et al. 2008) offers a solid framework for structuring different processes and for designing experiments.
General conclusions
The key message of our research is that significant genetic adjustments to increased temperature in the context of global warming can take place in our model system, the waterflea Daphnia, in a short time frame of a few months or years. We found evidence for thermal micro-evolution in life-history traits and competitive ability in a keystone freshwater zooplankton species that occurs on an ecologically relevant time scale (i.e., within one growing season). Given this information, we emphasize the need to incorporate evolutionary responses in models that aim at predicting the responses of populations and communities to global warming (see also Peck et al. 2009). We further show that evolution has the potential to profoundly influence ecological processes by impacting the relative importance of local and regional dynamics, which highlights the importance of the perspective of an evolving metacommunity for further research on the biological consequences of global warming for freshwater ecosystems (e.g., Urban and Skelly 2006; Urban et al. 2008; Gilman et al. 2010).
Funding
K. U. Leuven Research Fund projects (grants GOA/2008/06 and PF/2010/07); Fund for Scientific Research (FWO) (project G.0419.08); EU project EURO-LIMPACS; IWT fellowship (to W.v.D. and A.G.); L.O. is a postdoctoral researcher of the FWO.
References
Author notes
From the symposium “A Synthetic Approach to the Response of Organisms to Climate Change: The Role of Thermal Adaptation” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 3–7, 2011, at Salt Lake City, Utah.