-
PDF
- Split View
-
Views
-
Cite
Cite
Ruth Callaway, Nicolas Desroy, Stanislas F. Dubois, Jérôme Fournier, Matthew Frost, Laurent Godet, Vicki J. Hendrick, Marijn Rabaut, Ephemeral Bio-engineers or Reef-building Polychaetes: How Stable are Aggregations of the Tube Worm Lanice conchilega (Pallas, 1766)?, Integrative and Comparative Biology, Volume 50, Issue 2, August 2010, Pages 237–250, https://doi.org/10.1093/icb/icq060
Close - Share Icon Share
Abstract
Dense aggregations of tube-worms can stabilize sediments and generate oases for benthic communities that are different and often more diverse and abundant than those of the surroundings. If these features are to qualify as biogenic reefs under nature-conservation legislation such as the EC Habitats Directive, a level of stability and longevity is desirable aside from physical and biological attributes. Lanice conchilega (Pallas, 1766) is widely distributed around the European coast and aggregations of this tube-dwelling polychaete are known to have a positive effect on the biodiversity of associated species in inter- and sub-tidal areas. This increases the value of L. conchilega-rich habitats for higher trophic levels such as birds and fish. However, L. conchilega is currently not recognized as a reef builder primarily due to uncertainty about the stability of their aggregations. We carried out three studies on different spatial and temporal scales to explore a number of properties relating to stability: (1) Individual aggregations of L. conchilega of ∼1 m2 were monitored for up to 1 year, (2) records of L. conchilega from a 258-ha area over a 35-year period were analyzed, (3) the recovery of a population of L. conchilega subjected to disturbances by cultivation of Manila clams (Ruditapes philippinarum) was followed over 3 years. The studies provided evidence about the longevity of L. conchilega aggregations, their resistance to disturbance, their resilience in recovering from negative impact and their large-scale persistence. The results showed that populations of L. conchilega were prone to considerable fluctuation and the stability of aggregations depended on environmental factors and on recruitment. The tube-worms proved to be susceptible to disturbance by cultivation of Manila clams but demonstrated the potential to recover from that impact. The long-term monitoring of a large L. conchilega population in the Bay of Mont Saint Michel (France) indicated that aggregations can persist over many decades with a constant, densely populated core area and an expanding and contracting more thinly populated fringe zone. The stability of aggregations of L. conchilega and the structures they form do not unequivocally fit the currently accepted definition of a reef. However, given L. conchilega’s accepted reef-like potential to influence diversity and abundance in benthic communities, we suggest clarifying and expanding the definition of reefs so that species with records of significant persistence in particular areas and which otherwise meet expectations of reefs are included within the definition.
Introduction
Tube-building worms such as Sabellaria alveolata, Serpula vermicularis, or Ficopomatus enigmaticus can develop dense aggregations of intertwined tubes giving rise to large structures that are considered to be biogenic reefs (Fornos et al. 1997; Dubois et al. 2002; Poloczanska et al. 2004). Such reefs induce the stabilization of sediments (Murray et al. 2002) while the tubes of the organisms themselves provide complex structural habitats for attachment of sessile organisms (Dubois et al. 2006) and refuge or a food source for vagile vertebrates and invertebrates (Bruschetti et al. 2009). Reef structures can therefore be seen as biodiversity hotspots where species diversity deeply contrasts with that of surrounding sediments (Dubois et al. 2002). Due to their high-density polychaete reefs can also be seen as large biological filters and they play a significant role in trophic webs (Bruschetti et al. 2008; Dubois et al. 2009). Biogenic reefs are consequently of high importance to the ecological functioning of the habitats and areas in which they are found and it is for this reason that reefs are of particular interest to nature conservation. As a result, the conservation status of biogenic reefs is recognized by legislative regulations such as the EC Habitats Directive (Council Directive EEC/92/43 on the Conservation of Natural Habitats and of Wild Fauna and Flora), which lists reefs as a marine habitat to be protected by the designation of Special Areas of Conservation (European Commission DG Environment 2007). A number of biogenic reef types are also listed as threatened or declining by OSPAR (Ospar Convention for the Protection of the Marine Environment of the north–east Atlantic.) (Ospar Commission 2006).
According to the EC Habitats Directive “Reefs can be either biogenic concretions or of geogenic origin. They are hard compact substrata on solid and soft bottoms, which arise from the sea floor in the sublittoral and littoral zone. Reefs may support a zonation of benthic communities of algae and animal species as well as concretions and corollogenic concretions” (Council Directive EEC/92/43 on the Conservation of Natural Habitats and of Wild Fauna and Flora). Biogenic concretions are further defined as: concretion, encrustation, corallogenic concretions, and bivalve mussel beds originating from dead or living animals, i.e. biogenic hard bottoms which supply habitats for epibiotic species. For the North Atlantic region, polychaetes such as Sabellaria spinulosa, S. alveolata, and S. vermicularis, the bivalves Modiolus modiolus and Mytilus sp. as well as cold-water corals are specified as examples of characteristic reef forming species.
More detailed interpretations of this definition suggest that reefs should meet certain physical, biological, and temporal attributes (Holt et al. 1998; Hendrick and Foster-Smith 2006), with an expectation that they should be discrete, elevated structures covering a substantial area. Reef-builders should bind sediment and replace existing substratum with a new one and be colonized by a different benthic community than that occurring in the surrounding area.
In addition, reefs are also considered to be stable and to have a proven longevity since it is expected that a long-lived, stable biogenic concretion would have greater value in respect to conservation than would an otherwise comparable habitat of an ephemeral nature. Nevertheless, it is generally accepted that many reef-building organisms are to a certain degree ephemeral. Some beds of Mytilus edulis in the Wadden Sea, for example, may occupy the same sites for decades while others are short-lived (Herlyn et al. 2008). Similarly, a broad-scale UK-wide study showed that S. alveolata aggregations can appear and disappear at sites at a range of time scales from inter-annual to multi-decadal (Frost et al. 2004). In the case of S. spinulosa, longevity has been used as justification by the UK Biodiversity Group to differentiate between encrusting colonies of the species and the more upright morphology of the reefs. The group noted that crusts are not considered to constitute true S. spinulosa reef habitats because of their ephemeral nature, stating that they do not provide a stable biogenic habitat enabling associated species to become established in areas where they otherwise would be absent (UK Biodiversity Group 1999). Despite this, S. spinulosa, S. alveolata, and M. edulis are all specifically recognized as biogenic reef builders under the EC Habitats Directive.
This article focuses on the stability and longevity of aggregations of the tube-building sand mason worm L. conchilega. It is a terebellid polychaete (Figs. 1 and 2) that can form aggregations in coastal areas to a depth of ∼100 m, reaching densities of several thousand individuals per meter square (Hertweck 1995; Ropert and Dauvin 2000). It is an amphiboreal species found on all coasts of Europe and in both the Atlantic and the Pacific, but it is absent from arctic waters (Holthe 1978). Their tubes have a diameter of ∼0.5 cm and are up to 65-cm long (Ziegelmeier 1952), consisting of sand particles attached to an inner thin organic layer. The anterior end of the tube protrudes above the surface of the sediment by 1–4 cm and it has a fringe made of single-strand “sand hair”. Lanice conchilega can switch between deposit feeding and suspension feeding (Buhr 1976). The larvae are planktonic and were termed “aulophore” by Kessler (1963) because of their transparent tube that is used as a floating device.
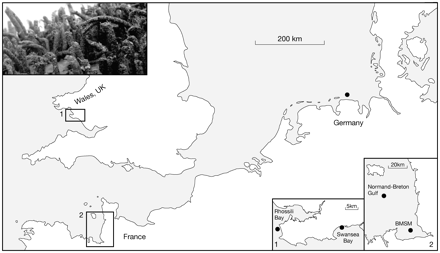
Location of study sites (filled circle) inhabited by L. conchilega. Study A: “Small scale stability of L. conchilega” was carried out at sites in the German Wadden Sea and in Wales, UK (1). Study B: “Long-term stability” was carried out in the Bay of Mont Saint Michel (BMSM), France (2). Study C: “The recovery of L. conchilega aggregations impacted by clam cultivation” was carried out in the Normand-Breton Gulf (France) (2).
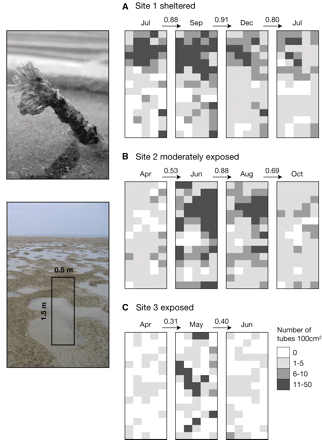
Small-scale distribution of L. conchilega within fixed 0.5 × 1.5 m plots at three differently exposed sites. Densities of L. conchilega tubes in 100-cm2 grid cells are shown. (A) Site 1 was in the Wadden Sea (Germany) and monitored four times over 1 year, (B) Site 2 was in Swansea Bay (Wales, UK) and monitored over 7 months. (C) Site 3 was in Rhossili Bay (Wales, UK) monitored over 3 months. The cosine similarity index between consecutive monitoring dates is given. One plot per site is shown. Images on the left show a single L. conchilega tube (top) and L. conchilega aggregations with a schematic monitoring plot (bottom).
In recent years, considerable efforts were made to assess the value of dense aggregations of L. conchilega for the inhabited environments. Once densities reach about 500 individuals meter square the tubes start consolidating the sediment and create a mound-and-trough topography (Rabaut et al. 2009). Aggregations of tubes were shown to provide niches for a different and generally more species-rich and abundant faunal community than the adjacent tube-free sands in both inter- and sub-tidal areas (Rabaut et al. 2007; Van Hoey et al. 2008). This has a positive knock-on effect for birds and fish (Godet et al. 2008; Rabaut et al. 2010).
Despite demonstrating many features considered characteristic of biogenic reefs, L. conchilega aggregations were specifically excluded from a review of reefs for conservation management by Holt et al. (1998) due to their assumed instability. Since then, aggregations of L. conchilega have been described as ephemeral in the Wadden Sea after a population was eradicated by low winter temperatures (Zühlke 2001), and Rabaut et al. (2009) discussed the lack of long-term data to estimate their temporal characteristics, emphasizing the lack of knowledge about their stability and longevity.
The aim of this article is to address the gap in knowledge regarding the longevity of aggregations of L. conchilega. We hypothesized that the stability of L. conchilega aggregations differs between different spatial and temporal scales, i.e., that individual aggregations on a scale of square meters may be short lived and ephemeral, but that the presence of the tube-worm is more persistent on larger spatial and temporal scales. We further hypothesized that aggregations of L. conchilega have the potential to recover from disturbance. We analyzed our studies in view of particular stability properties adapted from Grimm et al. (1999).
Constancy: the duration of a system to remain essentially unchanged, i.e., how long do aggregations of L. conchilega exist without interruption? How old are individual aggregations?
Resistance: the capability of a system to remain unchanged despite the presence of disturbances which could potentially change the system, i.e., what is the capacity for L. conchilega aggregations to remain unaffected in the presence of natural or anthropogenic disturbances such the harvesting of bivalves?
Resilience and Elasticity: this refers to the property to return to a reference state after a temporary disturbance and to the speed of recovery. Resilience is also defined as the capacity of a system to renew and sustain specified conditions or processes in spite of exogenous disturbances or changes in driving forces (Carpenter and Folke 2006), i.e., are aggregations of L. conchilega returning to their original state after being disturbed? How quickly do aggregations recover (elasticity)?
Persistence: the property of a system to exist over long periods of time, where the ecological system and disturbances affecting the system are seen as a unit. In contrast to constancy, which is defined as the continuous presence of a feature, persistence allows for the intermittent absence of the relevant population, community or other feature, if it recovers at some point, i.e., do aggregations of L. conchilega exist over long periods of time with phases of continuous presence alternating with phases of disturbance, or even absence, and recovery?
Three studies on different spatial and temporal scales were carried out to investigate these properties of stability.
Individual aggregations of L. conchilega of ∼1 m2 were monitored for up to 1 year.
Records for L. conchilega on a 258-ha area over a 35-year period were analyzed.
The recovery of a population of L. conchilega between disturbances by cultivation of Manila clams (R. philippinarum) was followed over 3 years.
Material and methods
Small- and large-scale studies on the stability of L. conchilega aggregations were carried out at inter-tidal sites in France, Germany, and the United Kingdom (Fig. 1).
Small-scale stability of L. conchilega
The constancy of individual aggregations of L. conchilega was studied at three sites subjected to different degrees of exposure (Fig. 1). In order to evaluate the stability of individual aggregations, plots of 0.75 m2 were marked at the sites (Site 1, n = 3; Site 2, n = 2; Site 3, n = 2). Because of the low number of replicates this study is limited. It provides broad insight into the subject and we were cautious not to over-interpret the data and only report unambiguous trends. Tube tops were counted in 100-cm2 cells arranged in a 15 × 5 grid. The number of tube-tops is highly correlated with the number of individuals burrowed in the sediment (Ropert and Dauvin 2000; Strasser and Pieloth 2001; Zühlke 2001; Callaway 2003; Bendell-Young 2006) and the error associated does not exceed 3% (Ropert 1999). Adult and juvenile tube-worms were distinguished by size. Tubes ∼5 mm in diameter were classed as adults, tubes 1–2 mm in diameter as juveniles. Each marked plot was monitored and counted repeatedly over time. Site 1 was monitored for 1 year and Sites 2 and 3 for 6 and 3 months, respectively, due to premature termination of the studies by storms washing away the plot markers.
Site 1 (sheltered): “Dornumer Nacken”, a sheltered sandflat in the German Wadden Sea (53° 41.73'N, 07° 28.30' E, from July 1994 to July 1995). The tidal range was ∼3 m and the area was protected from major hydrodynamic impacts by the backbarrier Island of Baltrum.
Site 2 (moderately exposed): Swansea Bay, a 15-km wide inlet east of the Gower Peninsula, South Wales, UK (51° 35.21' N, 03° 58.98' E, from April to October 1998). Tidal currents were strong due to a tidal range of ∼11 m, but the bay was sheltered from major exposure to waves by the headland “Mumbles Head”.
Site 3 (exposed): Rhossili Bay, a sandy beach at the western tip of the Gower Peninsula, South Wales, UK (51° 34.08' N, 04° 17.88' W, from April to June 1998). There the tidal range was ∼11 m and the area was exposed to high-energy waves generated in the Bristol Channel.
In order to check whether tubes were randomly distributed or significantly clustered, the index of dispersion was calculated. The index is based on the variance-to-mean ratio and chi-square statistic was used to test for significant difference from a theoretical Poisson distribution (Ludwig and Reynolds 1988; Blome et al. 1999).
Stability of an aggregation of L. conchilega over 35 years in the Bay of Mont Saint Michel (BMSM)
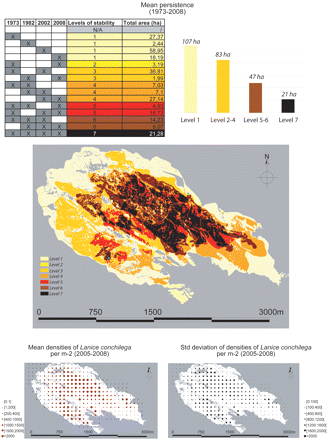
The presence of L. conchilega colonizing a 258-ha area was studied in the BMSM, France (48°39.33' N, 01°37.39' O) (Fig. 1). The beds of L. conchilega were mapped on a Geographical Information System (GIS) (Arcview 3.1) via photo-interpretation processing for 1973, 1982, 2002 and 2008 [L. conchilega aggregations can be detected on aerial photographs (1:10000 scale) from ± 250 ind m2, Laurent Godet personal observation]. Each date corresponds to a specific map and to a specific layer in the GIS. In addition, the spatial distribution of L. conchilega densities was examined within the area in 2005, 2006, 2007 and 2008. Densities were estimated by taking numerical pictures of three 0.25-m2 random quadrats in the middle of 362 regularly distributed stations (cells of 1 ha) covering the area. The number of intact tube-tops was counted on the pictures. Data from each year were superimposed as different layers with the GIS to distinguish between seven levels of stability (Fig. 3), resulting in a “stability map”.
Persistence and density of an aggregation of L. conchilega in the B MSM, France, from 1973 to 2008. Levels of stability were based on photo-interpretation of distribution maps of L. conchilega. In the table “X” correspond to the presence of an aggregation of L. conchilega as detected via the photo-interpretation process.
In order to compare densities with the stability of the aggregation two steps were taken:
The “stability map” was divided into 1-ha cells corresponding to the sample design used to assess the distribution, and for each cell a “stability index” was computed (stability index = percentage of the cell covering a stability level (levels 1–7) × level number).
For every cell, the mean and standard deviation of densities (2005–2008) were correlated with the “stability index” (R software).
Recovery of aggregations of L. conchilega impacted by cultivation of clams
In the Chausey archipelago, Normand-Breton Gulf, France (48°53.26' N, 01°49.17' O) (Fig. 1), Toupoint et al. (2008) demonstrated that cultivation of Manila clams (R. philippinarum) had strong effects on L. conchilega aggregations. Beyond this snap-shot study, we monitored the densities of L. conchilega in the same aggregation over 3 years and tested the potential resistance and resilience of the aggregation to this shellfish culture.
Clam cultivation is a highly mechanized activity in this region using tractor-driven machines during a production cycle which consists of three steps: (1) spat seeding; (2) no activities for 2 years to let the bivalves grow; and (3) harvesting (Toupoint et al. 2008). The highest impacts on L. conchilega correspond with steps (1) and (3). We tested the potential for recovery of L. conchilega over a 3-year period (2005–2007), expecting re-colonization of L. conchilega during step (2).
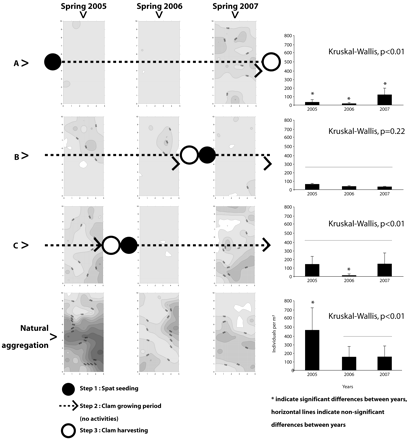
Each spring from 2005 to 2007, the spatial distribution of L. conchilega was examined in 1.28-ha plots (80 × 160 m) located in three areas of clam cultivation (A, B, and C) and, as a reference, in one area in which clams were not cultivated. The cultivation areas differed in terms of the timing of mechanically seeding and harvesting spat. Area A was seeded before spring 2005, area B was harvested and re-seeded between spring 2006 and spring 2007 and area C was harvested and re-seeded between spring 2005 and spring 2006 (Fig. 4).
Recolonization of L. conchilega in an area of Manila clam cultivation in the Chausey archipelago, Normand-Breton Gulf, France. The timing of seeding of spats and harvesting of clams is indicated. Three 80 × 160-m plots were monitored (Studies A–C) as was one reference plot (natural aggregation).
Each plot was divided into 50 cells (cell = 256 m2), and in each cell, three 0.25-m2 randomly placed quadrates were photographed. The number of tube-tops with sand-fringes was counted as a proxy for the number of individuals burrowed in the sediment.
The spatial distributions of L. conchilega were plotted by interpolating mean abundances and the linear kriging method using Surfer™ software. Differences between mean densities of L. conchilega from year-to-year were tested with nonparametric Kruskall–Wallis tests (KW) (R software). The assumptions of homoscedasticity were tested by Bartlett tests. In the case of significant global analysis, post hoc comparisons were performed through Tukey HSD tests or multiple comparison tests after KW (R software, package pgirmess).
Results
Small-scale and short-term stability of L. conchilega
Exposure of the sites and the success of recruitment of juveniles determined the similarity or dissimilarity of small-scale distributional patterns over time.
At the most sheltered site, Site 1 in the Wadden Sea, L. conchilega aggregations within the three 0.75-m2 plots remained generally at the same place over the 1-year period. The similarity index, indicating the relative spatial similarity between distributional patterns over time, was higher at Site 1 than at the more exposed Sites 2 and 3 in South Wales (Fig. 2). Lowest similarities, i.e., the least consistent aggregations, were found at the most exposed Site 3 (Rhossili Bay).
A striking difference between the sites was the recruitment success of juvenile L. conchilega. At the sheltered Site 1 no recruitment of juvenile L. conchilega occurred. At Sites 2 and 3, large numbers of juveniles settled in dense clusters between April and June, which changed the distribution pattern from random to significantly clustered (Index of dispersion, chi-square test, n = 75, P < 0.01). Many of the juveniles were lost shortly after the initial settlement and the degree of clustering decreased, particularly at Site 3 (Fig. 2).
The varying recruitment success had an impact on densities. Without recruitment of juveniles the density of L. conchilega at Site 1 decreased from 10.4 ± 10.8 to 6.5 ± 5.2 individuals 100 cm−2 (n = 225) over 1 year. At the moderately exposed Site 2, densities increased from 2.6 ± 2.0 to 4.2 ± 3.1 individuals 100 cm−2 (n = 150) from April to October, peaking at 21 ± 18.3 in May when juveniles had just settled. At the most exposed Site 3, densities increased from 0.4 ± 0.8 to 1.7 ± 2.7 individuals 100 cm−2 (n = 150) from April to June.
Stability of an aggregation of L. conchilega over 35 years in the BMSM
At maximum spread the L. conchilega population covered an area of 258 ha. The most persistent parts of the aggregation, present at all monitoring dates, covered 21 ha, i.e., 8% of the area of maximum spread. Fifty percent of the L. conchilega inhabited area showed medium levels of stability (2–6), whilst 41% was categorized as unstable with a stability level of 1. The most stable area was located at the core of the bed with stability decreasing with distance from the core (Fig. 3).
Densities and persistence of L. conchilega over time were positively correlated; the higher their densities the more persistent was their presence (linear model, adjusted R2: 0.2869, F-statistic: 118.1, 290 df, P < 0.01). Variance of densities and persistence were also positively correlated (linear model, adjusted R2: 0.1926, F-statistic: 70.42, 290 df, P < 0.01).
The recovery of aggregations of L. conchilega impacted by cultivation of clams
During the 2-year period between disturbances by seeding and harvesting of Manila clams, L. conchilega significantly re-colonized areas A and C (KW, P < 0.01) whereas no significant differences were observed in area B (KW, P = 0.22) (Fig. 4). The re-colonization pattern at the beginning of this period indicated that re-colonization took place from the periphery of the clam areas. However, L. conchilega did not reach densities as high as in the reference area in any of the three areas of clam cultivation.
Discussion
Our small- and large-scale studies showed that the stability of L. conchilega aggregations depended considerably on environmental factors and recruitment. The small-scale study, for instance, indicated that the stability of aggregations was determined by exposure of the site and by success of recruitment. In the larger study plots of the Chausey archipelago, L. conchilega was found to be susceptible to disturbance by Manila clam cultivation but had the potential to recover to a degree, while the long-term monitoring of the L. conchilega population in the BMSM indicated that under suitable conditions aggregations can persist over many decades.
Lanice conchilega is widely distributed and appears to tolerate a range of levels of exposure, although dense aggregations seem to form under more sheltered conditions (Hertweck, 1995). Our studies suggest that the level of exposure is also negatively correlated with the constancy of tube aggregations. This is likely to be a reflection of the ease of recruitment since juveniles of the tube-worm in particular are likely to be affected by severe movements of sediment (Zühlke and Reise, 1994).
Larval recruitment is paramount for the renewal of L. conchilega aggregations. Similar to other colony-forming invertebrates, juveniles of L. conchilega settle in close vicinity to their conspecific adults and it appears that hydrodynamic changes generated by biogenic mounds induce the pelagic larvae to settle on existing aggregations (Callaway 2003, Rabaut et al. 2009). Our studies showed that recruitment can fail over an entire year, which is most likely due to unfavourable environmental conditions with high mortality rates of the planktonic larvae or unsuitable conditions for the settling of juveniles. High year-to-year variation in recruitment success is known from other tube-dwellers such as S. alveolata where episodic events of massive settlement alternate with the lack of larval supply (Wilson, 1971, Dubois et al., 2007). It is possible that Sabellaria reefs are less susceptible to the lack of rejuvenation for a few years due to their more durable tubes and their longevity. They reach on average an age of 3–4 years (Caline et al. 1992), while the life span of L. conchilega is only ∼1–2 years (Beukema et al. 1978). Our study showed that a year without juvenile recruitment resulted in decreasing abundance of L. conchilega, but the population did not collapse. This suggests that aggregations of L. conchilega can survive years of failed recruitment, in particular when overall densities of the tube-worm are high. However, given their limited life span, recurrent failure of recruitment for 2 or 3 years should lead to the population’s demise.
Stability properties
The three studies gave insight into various stability properties.
Constancy
Our studies showed that individual aggregations may be either, short-lived or constant over long periods of time. Aggregations demonstrated the potential to remain constant for at least 1 year, substantiating reports of similar time-spans for L. conchilega mounds (Carey 1987). However, on a broader spatial scale, L. conchilega have remained continuously present in specific areas for much longer. In the B MSM, areas with high densities of L. conchilega remained constant for the past two decades (Godet et al. 2008).
Resistance
Our results suggest that despite their ability to rapidly rebuild and repair the tube (Nicolaidou 2003), L. conchilega does not necessarily resist sediment disturbance. The direct physical disturbance caused by seeding and harvesting of Manila clams negatively affected the presence of L. conchilega, with the most damaging practice being the scraping of the surface sediment layer during harvesting (Toupoint et al. 2008). The cultivation of clams not only has drastic effects on the total abundance of L. conchilega, but also modifies their spatial pattern and alters the associated benthic assemblage (Toupoint et al. 2008). The resulting alternation of habitat has knock-on effects for higher trophic levels with the area losing its attractiveness as a feeding ground for Oyster catchers (Haematopus ostralegus) (Godet et al. 2009). In sub-litoral regions also, suction dredging of Manila clams was found to have an equally negative effect on numbers of L. conchilega, although after 7 months the initially severe impact was hardly detectable (Kaiser et al. 1996).
Aggregations of L. conchilega were shown to possess some resistance to trawling when only several consecutive trawls resulted in a negative effect on numbers of L. conchilega (Rabaut et al. 2008). However, the associated fauna of the tube-worm was shown to change significantly after a one-off experimental trawl in the inter-tidal zone. Generally, species associated with L. conchilega seemed to be either vulnerable to beam trawling or they were negatively associated with L. conchilega under undisturbed conditions and increased in density after disturbance by beam trawling. This was found to be true for sub-tidal areas where close associates of L. conchilega, such as the polychaetes Eumida sanguinea, Phyllodoce mucosa and Eteone longa as well as some other co-occurring species (e.g., Abra alba and Kurtiella bidentata) were shown to be significantly impacted by trawling (Vanaverbeke et al., 2009). Densities of opportunistic species such as the polychaetes Capitella sp., Heteromastus sp. and Notomastus sp., which were considered to be negatively associated with L. conchilega, increased significantly shortly after passage of the beam trawl. Further, it has to be considered that the associated fauna of L. conchilega in many sub-tidal areas, for example along the North Sea coast, is most probably skewed to more resistant species given that more sensitive ones disappeared due to intensive fishing activities, which shifted the baseline of the benthic community structure (Callaway et al. 2007, Reiss 2009). This indicates that L. conchilega itself may have some resistance to intermediate beam trawling pressure, but that the integrity of the associated species community is more vulnerable.
Other anthropogenic activities have been found to affect L. conchilega through increased input of particles into the system. Sludge disposal of dredged material in the Weser estuary, for example, caused a strong decline in L. conchilega densities (Witt et al. 2004). The polychaete was absent from the disposal area as was its associated macrofauna, leading to an overall decrease in benthic diversity. Similarly, aquaculture operations with large-scale introductions of bivalve species, such as M. edulis, are changing the particulate composition of the environment through the production of pseudo-faeces and alterations in the hydrodynamic regime, and they have the capacity to destroy pelagic larvae (Davenport et al. 2000, Lehane and Davenport 2002). The development of M. edulis aquaculture along a coast may therefore have far reaching consequences for the renewal of intertidal L. conchilega aggregations, as well as for other macrofaunal species with pelagic larval stages.
While L. conchilega appears to be affected by high levels of disturbance of their sedimentary environment, it is even less resistant to a natural disturbance: low temperatures. The total demise of L. conchilega along large stretches of the Wadden Sea coast was the result of particularly cold winters (Buhr 1981; Strasser and Pieloth 2001). Losses were also reported at several locations in the UK following the severe winter of 1962–1963 (Crisp 1964). Lanice conchilega is obviously not able to resist this disturbance, but is able to re-colonize the lost ground.
Resilience and elasticity
The resilience of L. conchilega aggregations depends on the ability of larval and postlarval organisms to settle in areas where aggregations of tubes suffered some form of disturbance and were decimated or eradicated.
The small-scale distributional patterns of L. conchilega did not suggest much redistribution over time and given its sessile life style, this species may not be prone to prolific postlarval migration. However, in two of the three areas disturbed by cultivation of Manila clams measurable re-colonization took place after 1 or 2 years. The results tally with other descriptions of L. conchilega’s re-colonization strategy, which appears to start with few adults migrating into an area (Strasser and Pieloth 2001; Zühlke 2001). These relatively low numbers of postlarval immigrants provide a holdfast for juveniles and initiate a period of accelerated colonization. Also, juveniles may re-colonize an area by adhering to hard substratum such as shells of dead bivalves, rather than attaching to tubes of adult conspecifics (Herlyn et al. 2008). If the environmental conditions are favorable, juveniles simply settle directly into the sediment (Strasser and Pieloth 2001).
The duration of recovery, i.e., the elasticity, appears to range between 1 and 4 years (Beukema 1990; Heuers et al. 1998; Zühlke 2001). In our study, the L. conchilega population in the area of clam cultivation did not reach densities seen at the undisturbed reference site, suggesting that the interval between the seeding and harvesting of Manila clams was insufficient to allow full recovery.
Persistence
“Persistent” is probably the most appropriate term to describe aggregations of L. conchilega. Although L. conchilega may not resist disturbances particularly well, and their numbers may be regularly decimated by the absence of successful recruitment or by anthropogenic and natural disturbances, L. conchilega demonstrates remarkable stamina and success in re-colonizing areas that provide them with a suitable habitat (see “Resilience and elasticity” section).
The 35-year study in the B MSM demonstrated that some aggregations of considerable spatial dimension can persist over decades. The study also suggests that areas of high density of tubes are more persistent than are sparsely populated ones.
A persistent core area with high L. conchilega densities was surrounded by a less-dense and less-persistent peripheral area of the aggregation. Over time, the development of the population resembled a heartbeat: the space L. conchilega occupied contracted and expanded around a persistent core. Hertweck (1995) described very similar distributional patterns to the ones found here for the BMSM. The L. conchilega population he mapped in the Wadden Sea covered several square kilometers, with high densities of up to 10,000 individuals per square meters in central parts and less dense fringe areas with about 100 L. conchilega per square meters.
Given the preference of juvenile L. conchilega to settle close to adults (Callaway 2003), dense aggregations may benefit from self-amplification. The denser the aggregation, the higher the probability of successful rejuvenation. It has to be mentioned though that in our small-scale study the recruitment pattern was the reverse: the dense aggregation did not enjoy larval settlement while the sparsely populated ones did.
Lanice conchilega: reef or not reef?
Biogenic reefs provide important services for marine ecosystems and stable reef structures are likely to be more effective and of greater value than are transient ones (Holt et al. 1998; Thrush and Dayton 2002). They are also more manageable in terms of conservation.
Lanice conchilega does not intuitively fit the concept of a reef, even when tube densities are high and they create a characteristic mound-and-trough topography. However, in terms of effects on the benthic fauna aggregations of L. conchilega arguably play a reef-like role similar to other biogenic reefs such as S. alveolata. Aggregations of L. conchilega alter the community structure of the benthic fauna and potentially enrich biodiversity and increase abundance (Zühlke 2001; Callaway 2006; Rabaut et al. 2007; Van Hoey et al. 2008). Generally, the tube structures provide havens that allow species inhabiting the surrounding sediments to establish localized higher densities, with few species being exclusive to the aggregations. This is similar to reefs of S. alveolata in which the unique nature of the associated community is not related to the presence of particular species but is due to the juxtaposition of species belonging to surrounding communities (Dubois et al. 2002). The reef-forming tube-worm F. enigmaticus also alters the abundance of macro-faunal species present in the surroundings, which respond to the changed conditions within the reefs (Schwindt and Iribarne 2000).
Due to the enriched associated infauna in L. conchilega aggregations, they are of great importance for higher trophic levels such as birds and fish (Godet et al. 2008; Rabaut et al. 2010).
Our studies have shown that aggregations of L. conchilega are not stable in the sense of being constantly present with continuously high tube densities over long periods of time. On the contrary, populations are prone to considerable fluctuation as they are vulnerable to failure of recruitment as well as to natural and anthropogenic disturbances. Thus, the value of L. conchilega’s ecological service to a region will vary with the level of disturbance a population endures.
The stability of aggregations of L. conchilega lies in their resilience and elasticity, i.e., they recover predictably from disturbances and have proved capable of re-colonizing lost ground in 1 to 4 years (Beukema 1990; Heuers et al. 1998; Zühlke 2001). The associated fauna of L. conchilega is equally, if not more resilient than is L. conchilega itself (Rabaut et al. 2008). This flexible response to disturbance ensures their persistence in suitable habitats over decades, as shown for the population in the BMSM.
Whether or not L. conchilega is a reef-builder cannot be answered unequivocally. In terms of its service to biodiversity, L. conchilega can certainly be regarded as a reef-building polychaete. The key question is whether the persistent nature of L. conchilega aggregations, with its phases of disturbance and recovery, satisfies the criterion of longevity. We suggest that L. conchilega-rich areas with a proven record of decadal persistence should be regarded as a type of reef.
Conclusions
Our studies showed that, on the one hand, the size and density of L. conchilega aggregations varies considerably over time. On the other hand, L. conchilega is resilient and has a considerable potential to recover from disturbances. As a result, large areas are persistently inhabited by L. conchilega over decades. Given the known importance of L. conchilega for the biodiversity and community composition of a habitat, it can be assumed that these persistent aggregations provide reef-like services to the area over long periods of time. We therefore suggest reconsidering the conservation status of species such as L. conchilega, which have a proven benefit to marine environments, but do not unequivocally fit into the current definition(s) of reefs. A possible solution is the clarification of the definition of a reef and to broaden its interpretation to include species with records of long-lasting persistence in particular areas.
Funding
French Marine Protected Areas Agency, the CNRS and the French Museum of National History. Federal Ministry for Education, Science, Research and Technology, Germany (BMBF) for project 03F0112A.
Acknowledgments
We thank the National Science Foundation (IOS-0938257), The Society for Integrative and Comparative Biology Division of Ecology and Evolution and the Division of Invertebrate Zoology, and the American Microscopy Society for supporting our research. This manuscript is partly the result of a workshop that took place in Dinard (France) in September 2009. We thank Steven Degraer and an anonymous reviewer for their valuable comments on an earlier version of the manuscript.
References
Author notes
From the symposium “Marine Ecosystem Engineers in a Changing World: Establishing Links across Systems” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 3–7, 2010, at Seattle, Washington.